Image Processing and Analysis Techniques for Microscopy
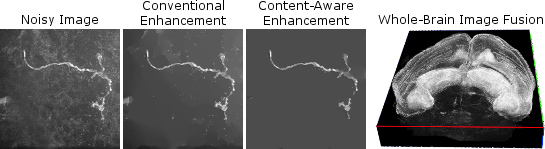
Microscopy imaging is a vital tool in a wide variety of biomedical applications. To address the growing need for robust, automatic image processing and analysis algorithms, our team is investigating model-based techniques for image enhancement and restoration and related uses in segmentation, cell tracking, and quantification. Recently, efforts have begun applying these ideas to whole-brain microscopic imaging in mouse models for epilepsy, neuron and glial cell imaging, and prostate cancer cells. This burgeoning area of the lab is developing through collaborations with faculty in enginneering, biomedicine, and biology. Image credits: Noisy neuron image provided by Scott Acton; brain image data provided by Jaideep Kapur.
Automatic Image Quality Comparison for Image Processing
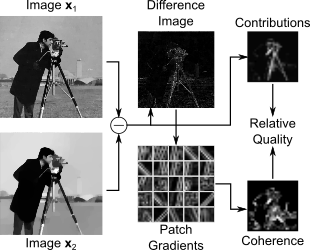
While humans are generally capable of assessing the quality (e.g., noise level, sharpness) of a series of images, perceptual valuations of image quality remain challenging for a computer, especially without access to a high-quality reference image. This research has produced an innovative approach for automatically comparing two or more images of the same object to determine which has the better quality, distinguishing noise and blur from salient image content. This approach results in improved image reconstructions and other forms of processing, like denoising, by enabling algorithms to be tuned or adjusted to produce the best possible quality image according to the automatic metric. We are investigating new approaches for improving image models and algorithms like dictionary-learning using this framework. We are also extending the framework to automatically identify artifacts introduced by typical image acquisition and reconstruction schemes in magnetic resonance imaging and other domains.
Motion-Robust, Rapid Magnetic Resonance Imaging
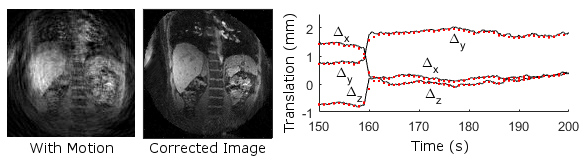
More powerful magnets, parallel imaging coils, and fast imaging protocols have made acquiring time-series of magnetic resonance images viable for the study of physiology in the brain, heart, liver, kidneys, and other organs, advancing both scientific research, and medical diagnostics and treatment. Coupling these exciting acquisition technologies with novel model-based reconstruction algorithms promises to increase spatial and temporal resolution, mitigate the presence of acquisition-related artifacts, and make imaging more comfortable for research subjects and patients. With greater resolution, such imaging can better localize activity in the multiple layers of the brain. Similarly, motion-robust, high-resolution cardiac imaging will enable detailed assessment of cardiac function while no longer requiring patients to hold their breath during scans. Dynamic imaging is also valuable for following progression of liver cancer and assessing kidney function. In all these cases, our lab is applying new model-based reconstructions featuring models for motion, structure, and dynamic variations. Future work on data-adaptive models, optimization techniques, and image quality metrics will continue to push the state-of-the-art. Working closely with clinicians in the University of Virginia Health System, these algorithms will be evaluated and applied in real clinical imaging applications, enabling our research to have significant immediate impact.


